The Asthma and Allergy Foundation of America (AAFA) is sharing this press release from AbbVie to bring you the latest research news quickly.
PRESS RELEASE
U.S. FDA Approves RINVOQ® (upadacitinib) to Treat Adults and Children 12 Years and Older with Refractory, Moderate to Severe Atopic Dermatitis
- Approval of two dose strengths (15 mg and 30 mg) supported by efficacy and safety data from one of the largest registrational Phase 3 programs in atopic dermatitis, with more than 2,500 patients evaluated across three studies[1]
- RINVOQ (upadacitinib) monotherapy or with topical corticosteroids met all primary and ranked secondary endpoints in atopic dermatitis pivotal studies[1-3]
- RINVOQ demonstrated significant improvement in itch (Worst Pruritus NRS ≥4) as early as week one, as well as significant improvements in skin clearance (EASI 75 and vIGA-AD 0/1) at 16 weeks, compared to placebo[1-3]
- RINVOQ also demonstrated significantly higher levels of skin clearance (EASI 90 and 100) at 16 weeks, compared to placebo[1-3]
NORTH CHICAGO, Ill. -- AbbVie (NYSE: ABBV) today announced the U.S. Food and Drug Administration (FDA) has approved RINVOQ® (upadacitinib) for the treatment of moderate to severe atopic dermatitis in adults and children 12 years of age and older whose disease did not respond to previous treatment and is not well controlled with other pills or injections, including biologic medicines, or when use of other pills or injections is not recommended.1 RINVOQ 15 mg once daily can be initiated in adults and children 12 years of age and older weighing at least 40 kg.1 In these children and adults less than 65 years of age who do not achieve an adequate response, the dose may be increased to 30 mg once daily.1
"Early in my career as an allergist, I saw how relentless the itch and rash could be for my patients with moderate to severe atopic dermatitis yet had limited options to offer those whose disease could not be adequately controlled with systemic therapy," said Thomas Hudson, M.D., senior vice president, research and development, chief scientific officer, AbbVie. "This additional approval for RINVOQ provides a once-daily oral option that can significantly improve the debilitating itch and skin symptoms of atopic dermatitis. It's also a proud moment for AbbVie as we continue our efforts to improve care in this disease state and other chronic, immune-mediated conditions."
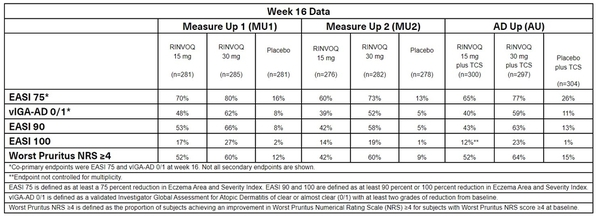
The FDA approval is supported by efficacy and safety data from one of the largest registrational Phase 3 programs for atopic dermatitis with more than 2,500 patients evaluated across three studies. Approximately 52 percent of the patients had prior exposure to systemic atopic dermatitis treatment. These studies evaluated the efficacy and safety of RINVOQ monotherapy (Measure Up 1 and 2) and with topical corticosteroids (AD Up), compared to placebo, in adults and children 12 years of age and older with moderate to severe atopic dermatitis.2-3
"Despite available therapies, many people with moderate to severe atopic dermatitis are caught in an endless cycle of itching and scratching," said Emma Guttman-Yassky, M.D., Ph.D., Waldman Professor and System Chair of Dermatology at the Icahn School of Medicine at Mount Sinai in New York City.* "In clinical trials, upadacitinib showed a robust response across skin and itch symptoms that may help evolve treatment goals for those who have not achieved adequate control of their disease. And as an oral pill with two dose strengths, upadacitinib is a welcome addition to the toolbox of clinicians who are striving to make a significant difference for their patients with moderate to severe atopic dermatitis."
Clinical Response at Week 161-3
- Across the three atopic dermatitis pivotal studies, RINVOQ (15 mg and 30 mg, once daily) monotherapy and with topical corticosteroids met all primary and secondary endpoints at week 16, with some patients achieving higher levels of skin clearance (EASI 90 and 100).
Itch Reduction1-3
- In all three studies, a significant improvement in itch (Worst Pruritus NRS ≥4) was observed as early as week one, compared to placebo.
Safety1-3
- Overall, the safety profile observed in patients with atopic dermatitis treated with RINVOQ 15 mg or 30 mg was similar to the safety profile observed in patients with rheumatoid arthritis. Other specific adverse reactions reported in atopic dermatitis patients included eczema herpeticum/Kaposi's varicelliform eruption.
- RINVOQ may cause serious side effects, including:
- Serious infections. RINVOQ can lower your ability to fight infections. Serious infections have happened while taking RINVOQ, including tuberculosis (TB) and infections caused by bacteria, fungi, or viruses that can spread throughout the body. Some people have died from these infections.
- Increased risk of death in people 50 years and older with at least 1 heart disease (cardiovascular) risk factor.
- Cancer and immune system problems. RINVOQ may increase your risk of certain cancers, including lymphoma, skin, and lung cancer, as these can happen. Current or past smokers are at higher risk.
- Increased risk of major cardiovascular events such as heart attack, stroke, or death in people 50 years and older with at least 1 heart disease (cardiovascular) risk factor, especially in current or past smokers.
- Blood clots. Blood clots in the veins of the legs or lungs and arteries can happen with RINVOQ. This may be life-threatening and cause death. This has happened more often in people 50 years and older with at least 1 heart disease (cardiovascular) risk factor.
Do not take RINVOQ if you are allergic to upadacitinib or any of the ingredients in RINVOQ.
Other serious side effects include serious allergic reactions, tears in the stomach or intestines and changes in certain laboratory test results.
Patient Access and Support
AbbVie is committed to helping people access RINVOQ and other medicines, including offering a patient support program and a co-pay card that may reduce out-of-pocket costs to as little as $5 per month for eligible, commercially-insured patients. For those with limited or no health insurance, AbbVie offers myAbbVie Assist, a patient assistance program that provides RINVOQ at no charge to those who qualify. More information about this assistance program can be found on www.AbbVie.com/myAbbVieAssist.
The Impact of Atopic Dermatitis
Atopic dermatitis is a chronic, relapsing inflammatory condition characterized by a cycle of intense itching and scratching that leads to cracked, scaly and oozing skin.4-6 It affects an estimated 7 percent of adults and 12 percent of adolescents in the U.S., with approximately 40 percent of adults experiencing moderate to severe disease.7-8 It manifests differently across individuals, with symptoms posing significant physical, psychological and economic burdens.4-5,9
"Every person with atopic dermatitis has a unique experience with their disease, and in turn, must have multiple options to choose from in their journey to find a treatment that meets their individual needs," said Julie Block, president and chief executive officer, National Eczema Association. "This approval is a significant milestone for our community, providing an additional therapy that may bring relief to those living with the devastating symptoms of moderate to severe atopic dermatitis."
About RINVOQ® (upadacitinib)
Discovered and developed by AbbVie scientists, RINVOQ is a selective JAK inhibitor that is being studied in several immune-mediated inflammatory diseases.10 Based on enzymatic and cellular assays, RINVOQ demonstrated greater inhibitory potency for JAK-1 vs JAK-2, JAK-3, and TYK-2.1 The relevance of inhibition of specific JAK enzymes to therapeutic effectiveness and safety is not currently known.
In the U.S., RINVOQ 15 mg and 30 mg is approved for use in adults and pediatric patients 12 years of age and older with refractory, moderate to severe atopic dermatitis whose disease is not adequately controlled with other systemic drug products, including biologics, or when use of those therapies are inadvisable.1 RINVOQ 15 mg is also approved in the U.S. for adults with moderately to severely active rheumatoid arthritis who have had an inadequate response or intolerance to one or more TNF blockers as well as adults with active psoriatic arthritis who have had an inadequate response or intolerance to one or more TNF blockers. In the EU, RINVOQ 15 mg is approved for the treatment of adults with moderate to severe active rheumatoid arthritis, adults with active psoriatic arthritis and adults with active ankylosing spondylitis. RINVOQ is also approved in the EU for adults (15 mg and 30 mg) and adolescents (15 mg) with moderate to severe atopic dermatitis.
Phase 3 trials of RINVOQ in rheumatoid arthritis, atopic dermatitis, psoriatic arthritis, axial spondyloarthritis, Crohn's disease, ulcerative colitis, giant cell arteritis and Takayasu arteritis are ongoing. 11-18
About AbbVie
AbbVie's mission is to discover and deliver innovative medicines that solve serious health issues today and address the medical challenges of tomorrow. We strive to have a remarkable impact on people's lives across several key therapeutic areas: immunology, oncology, neuroscience, eye care, virology, women's health and gastroenterology, in addition to products and services across its Allergan Aesthetics portfolio. For more information about AbbVie, please visit us at www.abbvie.com. Follow @abbvie on Twitter, Facebook, LinkedIn or Instagram.
*Emma Guttman-Yassky, M.D., Ph.D., is a researcher/consultant for AbbVie.
References
- RINVOQ® (upadacitinib) [Package Insert]. North Chicago, Ill.: AbbVie Inc.
- Guttman-Yassky E., et al. Once-daily upadacitinib versus placebo in adolescents and adults with moderate-to-severe atopic dermatitis (Measure Up 1 and Measure Up 2): results from two replicate double-blind, randomised controlled phase 3 trials. Lancet. 2021; 397(10290): 2151-2168. doi:10.1016/S0140-6736(21)00588-2.
- Reich, Kristian et al. Safety and efficacy of upadacitinib in combination with topical corticosteroids in adolescents and adults with moderate-to-severe atopic dermatitis (AD Up): results from a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2021; 397(10290): 2169 – 2181. doi:10.1016/S0140-6736(21)00589-4.
- Nutten S. Atopic Dermatitis: Global Epidemiology and Risk Factors. Ann Nutr Metab 2015;66(suppl 1):8–16.
- Weidinger S., et al. Atopic dermatitis. Nat Rev Dis Primers 4, 1 (2018). https://doi.org/10.1038/s41572-018-0001-z.
- University of Michigan Medicine. Atopic Dermatitis (Eczema). 2020. Available at: https://www.uofmhealth.org/hea...ry/hw216104#hw216107. Accessed on December 10, 2021.
- Silverberg JI. Public Health Burden and Epidemiology of Atopic Dermatitis. Dermatol Clin. 2017;35(3):283-289.
- Chiesa Fuxench ZC, Block JK, Boguniewicz M, et al. Atopic Dermatitis in America Study: A Cross-Sectional Study Examining the Prevalence and Disease Burden of Atopic Dermatitis in the US Adult Population. J Invest Dermatol. 2019;139(3):583-590.
- Eichenfield L.F., et al. Guidelines of care for the management of atopic dermatitis: section 1. Diagnosis and assessment of atopic dermatitis. J Am Acad Dermatol. 2014;70(2):338-351. doi:10.1016/j.jaad.2013.10.010.
- Pipeline – Our Science | AbbVie. AbbVie. 2019. Available at: https://www.abbvie.com/our-science/pipeline.html. Accessed on December 10, 2021.
- A Study Comparing Upadacitinib (ABT-494) to Placebo in Adults With Rheumatoid Arthritis on a Stable Dose of Conventional Synthetic Disease-Modifying Antirheumatic Drugs (csDMARDs) Who Have an Inadequate Response to csDMARDs Alone (SELECT-NEXT). ClinicalTrials.gov. 2021. Available at: https://clinicaltrials.gov/ct2/show/NCT02675426. Accessed on December 10, 2021.
- Evaluation of Upadacitinib in Adolescent and Adult Patients With Moderate to Severe Atopic Dermatitis (Eczema) (Measure Up 1). ClinicalTrials.gov. 2021. Available at: https://clinicaltrials.gov/ct2/show/NCT03569293. Accessed on December 10, 2021.
- A Study Comparing Upadacitinib (ABT-494) to Placebo and to Adalimumab in Participants With Psoriatic Arthritis Who Have an Inadequate Response to at Least One Non-Biologic Disease Modifying Anti-Rheumatic Drug (SELECT - PsA 1). ClinicalTrials.gov. 2021. Available at: https://clinicaltrials.gov/ct2/show/NCT03104400. Accessed on December 10, 2021.
- A Study to Evaluate Efficacy and Safety of Upadacitinib in Adult Participants With Axial Spondyloarthritis (SELECT AXIS 2). ClinicalTrials.gov. 2021. Available at: https://clinicaltrials.gov/ct2/show/NCT04169373. Accessed on December 10, 2021.
- A Study of the Efficacy and Safety of Upadacitinib (ABT-494) in Participants With Moderately to Severely Active Crohn's Disease Who Have Inadequately Responded to or Are Intolerant to Biologic Therapy. ClinicalTrials.gov. 2021. Available at: https://clinicaltrials.gov/ct2/show/NCT03345836. Accessed on Accessed on December 10, 2021.
- A Study to Evaluate the Safety and Efficacy of Upadacitinib (ABT-494) for Induction and Maintenance Therapy in Participants With Moderately to Severely Active Ulcerative Colitis (UC). ClinicalTrials.gov. 2021. Available at: https://clinicaltrials.gov/ct2/show/NCT02819635. Accessed on December 10, 2021.
- A Study to Evaluate the Safety and Efficacy of Upadacitinib in Participants With Giant Cell Arteritis (SELECT-GCA). ClinicalTrials.gov. 2021. Available at: https://clinicaltrials.gov/ct2/show/NCT03725202. Accessed on December 10, 2021.
- A Study to Evaluate the Efficacy and Safety of Upadacitinib in Subjects With Takayasu Arteritis (TAK) (SELECT-TAK). ClinicalTrials.gov. 2021. Available at: https://clinicaltrials.gov/ct2/show/NCT04161898. Accessed on December 10, 2021.
Contact(s)
Brittany Seagraves
+1 (224) 229-2144
Brittany.Seagraves@abbvie.com


Comments (0)