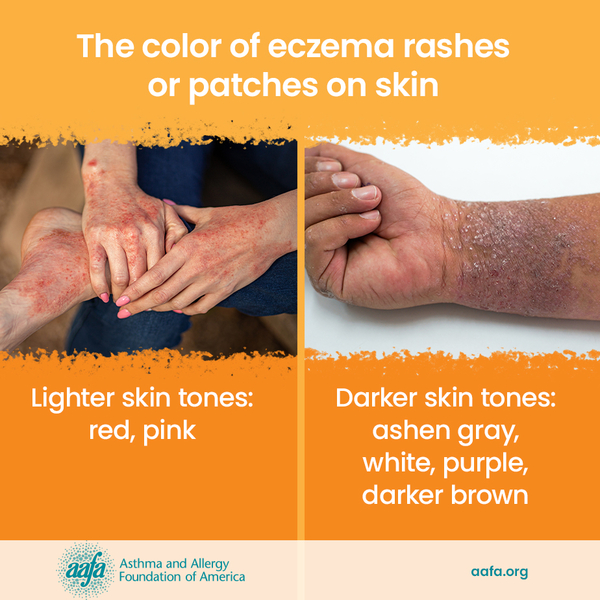
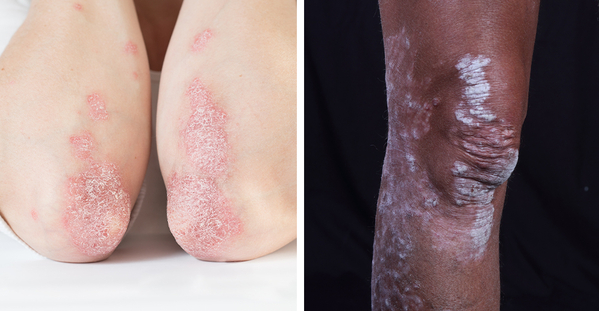
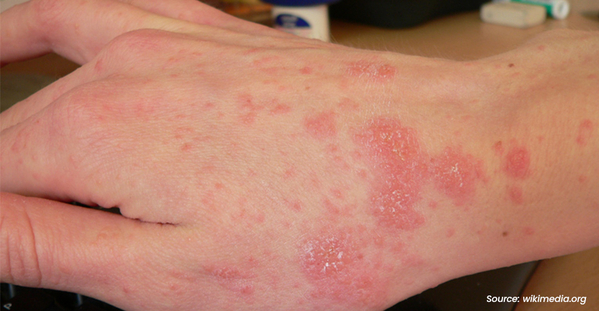
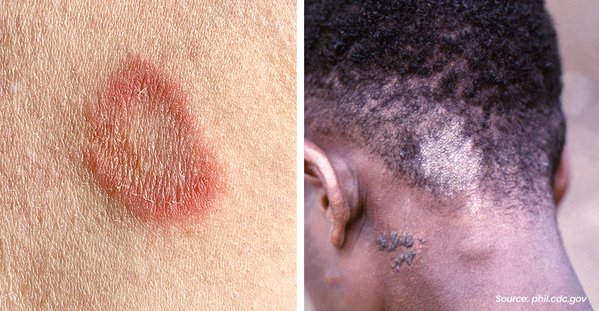
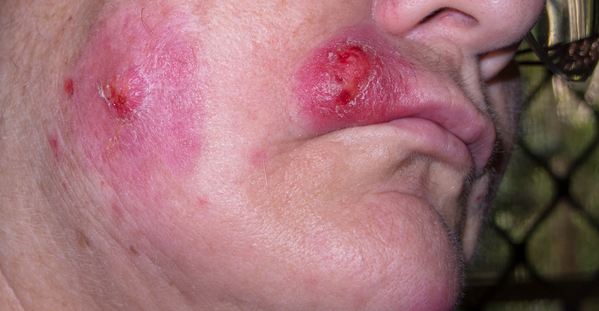
Eczema is a group of chronic skin conditions that cause your skin to become inflamed, dry, itchy, and irritated. The rash can be a different color than your normal skin tone. Atopic dermatitis (AD) is the most common type of eczema. If you or a family member has asthma and allergies, you have a higher chance of having AD. It affects nearly 10 million children and 17 million adults in the United States.
Living with AD can be challenging. Many people living with AD report symptoms like sore and painful skin, and poor sleep caused by itching. These physical symptoms can also impact mental health, social life, and overall well-being.
There is no cure for AD., but it can be managed through medical treatments like topical corticosteroids, non-steroidal topicals, and biologic treatments. AD can also be managed by avoiding known triggers and managing stress.
In 2024, the Food and Drug Administration (FDA) approved 2 new treatments for AD:
- ZORYVE® (a non-steroidal treatment that you put on your skin)
- EBGLYSS™ (a biologic)
Here is more information about these new treatments, how they work, and the research that supports their approval:
ZORYVE®
In July, the FDA approved ZORYVE cream for the treatment of AD in children and adults aged 6 and older. ZORYVE is a once-daily, steroid-free topical cream. It can be used on any location on the body affected by AD and can be used for as long as needed.
ZORYVE was previously approved for the treatment of seborrheic dermatitis and plaque psoriasis. ZORYVE works by blocking an enzyme called PDE4. Blocking this enzyme can help calm down the body’s nerves on the skin that cause you to itch and reduce inflammation caused by conditions like AD.
The approval of ZORYVE cream is based on positive results from several studies. In these studies, people with mild to moderate AD used ZORYVE cream or a placebo cream for 4 weeks.
The studies showed that ZORYVE helped many patients improve their skin. About 40% of people had clear or almost clear skin by the end of the 4 weeks.
The cream also helped reduce itching quickly. Some people felt better within 24 hours. More than 30% of participants had major itch relief after 4 weeks. Long-term studies also showed that many participants continued to improve. More than 60% had significant relief up to 56 weeks.
Very few participants reported adverse reactions. Most of the people who did report side effects said they had headaches, nausea, and minor skin irritation where they used treatment.
EBGLYSS™
In September, the FDA approved EBGLYSS for the treatment of moderate to severe AD in people aged 12 and older who weigh at least 88 pounds and who have AD that is not well-controlled with creams or ointments.
EBGLYSS is an injectable biologic treatment that can be used alone or with topical steroids. EBGLYSS works by blocking a protein in the body that causes too much inflammation.
Treatment starts with 2 injections at week 0 and week 2, followed by 1 injection every 2 weeks until week 16. After that, you can switch to a monthly maintenance dose.
The FDA approved EBGLYSS based on findings from 3 clinical trials that included more 1,000 people. In these studies, 38% of people who took EBGLYSS had clear or almost clear skin at week 16, compared to 12% of participants who did not receive treatment. One in 10 participants who took EBGLYSS saw these results in as early as 4 weeks. Of the people who experienced clear or almost-clear skin at week 16, most (77%) maintained those results at 1 year with monthly maintenance dosing.
Many participants also had itch relief with EBGLYSS. On average, 43% of people who took EBGLYSS felt itch relief at week 16, compared to 12% of participants who did not get the treatment. Of the people who felt itch relief at week 16, most (85%) still felt relief at 1 year of treatment with monthly maintenance dosing.
The most common side effects of EBGLYSS include redness, swelling, and inflammation of the eye and eyelid, minor skin irritation at the injection site, and shingles.
The recent FDA approvals of EBGLYSS and ZORYVE provide more treatment options for people living with eczema. Both treatments offer new ways to manage challenging symptoms like itching and irritation. With more treatment options available, people with AD are one step closer to getting relief and improving their quality of life. As always, talk with your health care provider to see if one of these treatments might be right for you.
Stay in the loop - get news and research updates straight to your inbox with our e-newsletters.
SIGN ME UP FOR UPDATES!